
National Bioethics Committee for Research
Health Research Institute
National Institutes of Health (NIH)

Routine Review Process
The National Bioethics Committee for Research reviews research on human subjects. All research (medical or social science) projects involving human subjects, whether as individuals or communities, including surveys, drug/device trial, the use of fetal material, embryos and tissues from alive or the recently dead done with
• International Funding specifically given for research done anywhere in Pakistan e.g. Research Advocacy Fund (RAF), DFID, USAID etc.
• Funded or supported by the Government of Pakistan.
• Any other research either done all over Pakistan or is a multi-province.
• Drug trials for registration.
shall be reviewed and approved by the NBC-R before a study is started.
The duration of approval for a study shall be valid to a maximum period of one (1) year after which a re-approval is required. This will be standard for any study submitted irrespective of its duration whether one (1), five (5) or more years. Any change in conditions that could affect the rights/autonomy/welfare of subjects or serious adverse event during a study must be informed for approval to continue the study. See More
In exceptional circumstance urgent/expedited review may be done by at least two or more members and the chairman for approval or disapproval. Such approvals shall be reported to the next meeting of NBC-R. The response letter to the applicant should mention that the decision is through expedited review. (This would be in special circumstance and not for convince).
Studies that do not require direct human subject participants e.g. chart review, already collected data for routine public health reasons etc, case reports may be exempted for review. But these studies and NBC-R exemption form would have to be submitted to the NBC-R to issue an exemption certificate. Such exemptions shall be reported to the next meeting of NBC-R.
An application for ethical review should be accompanied by:
- NBC-R ERC Application form with checklist.
- Research Protocol in standard format.
- One copy of informed consent in English and Urdu or any other local language of the population study.
- Questionnaire in English and Urdu administered during the study (if applicable).
- Head wise budget, and source of funding.
- CV of PI and Co PIs with justification as to how their qualification and experience makes them suitable for conducting the submitted research.
- List of ongoing projects in which currently the PI is involved.
- MOU from participating study sites if PI is from another institution.
- IRB approvals from all study sites in case of a multi-center study.
- Budget allocation with justification and allocation for different study sites.
- Material Transfer Agreement (MTAs) where applicable.
In case of Clinical trials additional requirements:
- Approval letters of Clinical Trial Sites by DRAP.
- A copy of Drug Brochure or any supplementary information enclosed (if applicable).
- Document related to the provision of health insurance of study participants.
- Notification of Data Safety and Monitoring Board (DSMB), if applicable. A CITI and GCP certification (In case of clinical trials).
- Declaration of Conflict of Interest (if any).
- Make a copy of this entire application for your files.
- NBC-R review will be sent to PI within eight weeks after submission. In exceptional circumstances expedited review will be done within 08 weeks owing to the COVID related studies burden.
- Once the review by NBC-R is sent to PI, it is expected that the PI must respond within 2-months, if no response from PI is received at the end of two months, a letter will be sent to PI stating ‘that if in the next 1-month the PI does not respond the study will be considered to be abandoned by PI’ and will be marked as ‘Null and Void’in the Submission Record.
Submission
Time line for the process
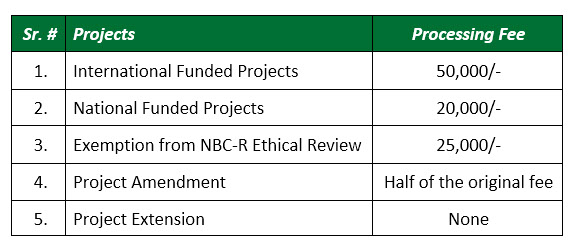
Fee Structure
National Bioethics Committee for Research (NBC-R), NIH (HRI),
Shahrah-e-Jamhuriat, G-5/2
Islamabad, Pakistan
Tel: +92-51-9224325, 9207386
Fax: +92-51-9216774
nbcpakistan@nih.org.pk